Combustion of Cyclohexane
Figure 1 shows the gravimetric and volumetric hydrogen H 2 densities for various hydrogen carriers. Cahier délève Activités Cours Exercices de la physique chimie pour le Tronc commun scientifique réalisé par prof.
Exemples C n H 2n.

. The results of the model have also been compared to species concentrations measured in a stirred reactor and a flow reactor. The minimum concentration of a particular combustible gas or vapor necessary to support its combustion in air is defined as the Lower Explosive Limit LEL for that gas. Download files CHEMKIN format.
The journal is dedicated to civil engineering aspects of the issues sources and programs that are either directly related to or can ultimately contribute to the production distribution and storage of energy. The maximum concentration of a gas or vapor that will burn in air is defined as the Upper Explosive Limit UEL. Cyclohexane is a cycloalkane with the molecular formula C 6 H 12Cyclohexane is non-polarCyclohexane is a colorless flammable liquid with a distinctive detergent-like odor reminiscent of cleaning products in which it is sometimes usedCyclohexane is mainly used for the industrial production of adipic acid and caprolactam which are precursors to nylon.
Melting point - the temperature at which a solid turns into a liquid. Hartley in Comprehensive Organometallic Chemistry 1982 39543iii Thermochemistry A bond dissociation energy of 213 kJ mol 1 has been determined from heats of combustion studies for the Pt IV CH 3 bond. The tables and figures below show how the boiling point changes with increasing carbon number up to C 33 for different kinds of hydrocarbons alcohols and carboxylic acids.
Air - Speed of Sound vs. More detailed definitions and examples of molecular structures of the different groups are given below the figures. Journal of Thermal Analysis and Calorimetry is a fully peer reviewed journal publishing high quality papers covering all aspects of thermal analysis calorimetry thermodynamics heat and energy.
Alcohols Butanol Isomers Iso-pentanol Alkanes 2-Methyl and n-Alkanes n-Dodecane. 806 This is very similar to the 223 kJ mol 1 determined in the same study for the Pt IV I bond. Since isomeric hydrocarbons must give the same mixture of CO 2 and H 2 O on complete combustion differences in their potential energy will be revealed by their heats of combustion.
The combustion of a stoichiometric mixture of fuel and oxidizer eg. As noted in the reaction energetics section isomers may have different potential energies reflecting the bond energies and strain in each. The journal publishes regular and special issues in twenty four issues every year.
43380 3340 18653 Monoolefins Ethylene. Below this level the mixture is too lean to burn. 43450 3385 18684 Methylcyclohexane.
Temperature - Speed of sound in air at standard atmospheric pressure with temperatures ranging -40 to 1000 o C -40 to 1500 o F - Imperial. 806 This is similar to the situation found for platinumII see. Ammonia has a very high hydrogen density and can either be used as a fuel for combustion systems without requiring a hydrogen extraction process or as a fuel in solid.
All candidate compounds other than pure hydrogen require energy to absorb and release hydrogen. The following types of papers are published. Acoustics - Room acoustics and acoustic properties decibel A B and C Noise Rating NR curves sound transmission sound pressure sound intensity and sound attenuation.
La combustion incomplète qui produit du carbone solide et de leau. The Journal of Energy Engineering reports on the scientific and engineering knowledge in the planning development management and finances of energy-related programs. Two moles of hydrogen and one mole of oxygen in a steel container at 25 C 77 F is initiated by an ignition device and the reactions allowed to complete.
Formule développée du cyclohexane. The detailed chemical kinetic mechanism has been validated by comparisons to experimentally measured laminar flame speeds and shock tube ignition delay times. Pour nommer un cycloalcane il suffit de rajouter le préfixe cyclo- au nom de lalcane linéaire ayant le même n sachant quil ne peut y avoir de cycle quavec n supérieur à deux.
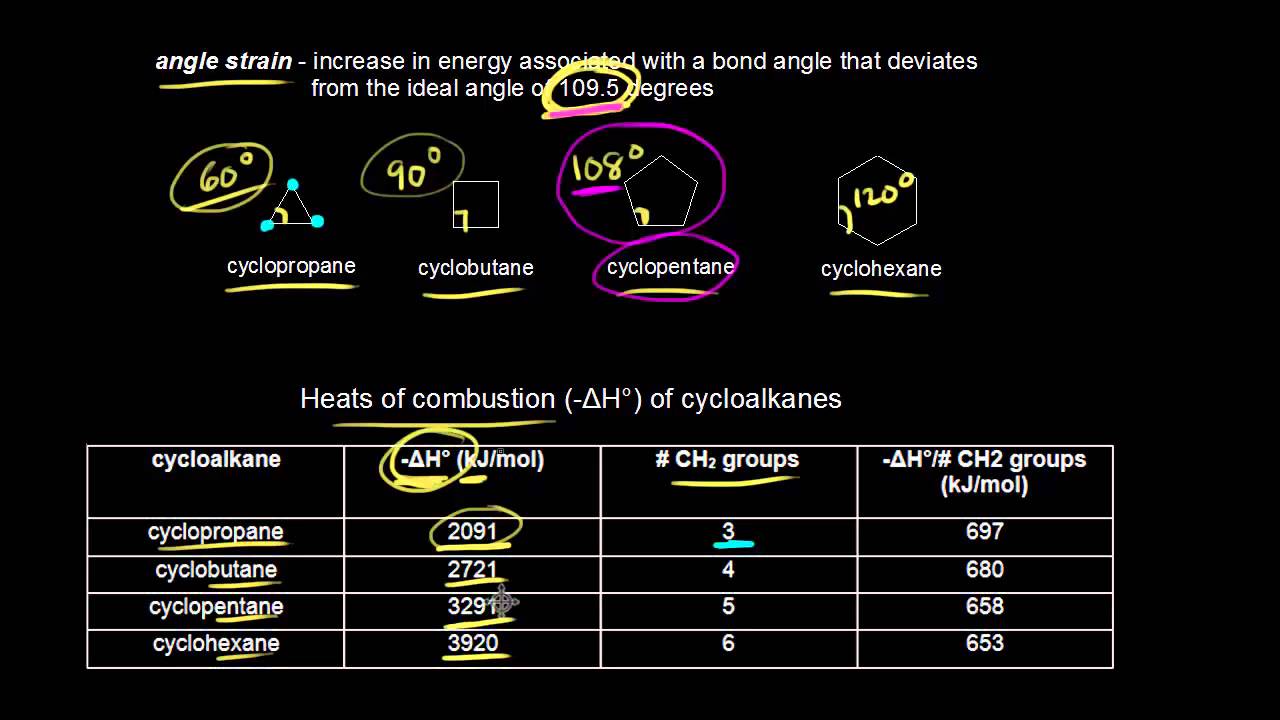
Stability Of Cycloalkanes Organic Chemistry Books Organic Chemistry Chemistry
0 Response to "Combustion of Cyclohexane"
Post a Comment